Abstract
Background: NLPHL is an uncommon variant of Hodgkin lymphoma (HL) accounting for about 4-6% of all HL cases. It has a distinct morphologic, immunohistochemical and clinico-pathological characteristics with CD20-positive "lymphocyte predominant cells". Although survival is better than classical HL, frequent relapses are common and transformation to aggressive non-Hodgkin lymphoma (NHL) may occur. Use of rituximab in smaller series over the past 5-8 years has shown promising results. We are reporting patient's presentation, management and outcome at our institution, one of the largest single institution data and first report from the Middle Eastern countries analyzed to date.
Design: All patients with NLPHL seen in our institution between January 2002 to May 2017 were included using our prospective lymphoma data base and Hospital Tumor Registry. Kaplan-Meir (KM) method was used to estimate 5-year progression free survival (PFS), defined as the time from diagnosis to disease relapse or progression or start of salvage due to primary treatment failure or death from any cause, whichever came first. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Staining for the expression of programmed death ligand-1 (PD-L1) is ongoing.
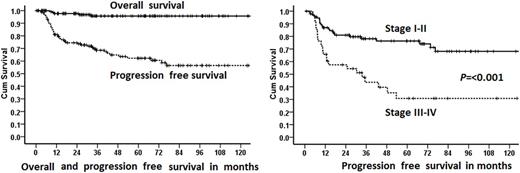
Results: We identified 145 patients; representing 6.8% of all the HL seen in our institution. There was a predominance of male patients, male: female ratio of 113:32 (3.5:1). Median age at diagnosis 20.5 years (range 4.7-79.3 years) and median follow-up 50 months (1-179). Stage at presentation was I:II:III:IV:unknown in 44:56:33:10:2 (30:39:23:7:1%). B-symptoms were present in 9 (6%), bulky disease in 15 (10.3%), spleen involvement in 19 (13%) and extranodal involvement in 21 (14.5%) patients. Primary treatment was excision / surgery alone in 8 (5.5%), radiation therapy (XRT) alone in 9 (6%) (after rituximab in 1), chemotherapy alone in 72 (50%), chemotherapy and XRT in 54 (37%) and 1+1 (1.5%) unknown and no treatment (lost to follow-up). Radiation after the first line chemotherapy was used as post-chemotherapy consolidation (already in complete remission (CR) in 33 (23%), to eradicate residual disease after partial remission (PR) in 17 (12%) and post-chemotherapy unknown in 4 (3%). Most common chemotherapy was adriamycin, bleomycin, vinblastin and dacarbazine (ABVD) in 119 (82%), ABVD/COPP (cyclophosphamide, vincristine, procarbazine, prednisone) in 2 (1.5%), Rituximab-CHOP (cyclophosphamide, adriamycin, vincristine, procarbazine, prednisone) in 3 (2%) and other in 2 (1.5%) patients. Rituximab was used with the first line (12 patients (8%)); with chemotherapy (11 patients) or with XRT (1 patient). Response to initial therapy was complete remission (CR) 107 (74%), partial response (PR) 11 (7.6%), no response/stable disease in 2 (1.4%), progressive disease (PD) in 13 (9%), pending response evaluation in 5 (3.4%) and not evaluated due to lost to follow-up/other reasons in 7 (5%). The first event was the persistent disease in 11 (7.6%), PD in 16 (11%), relapsed disease in 20 (14%), treatment related mortality (TRM) in 1 and death due to other cause in 1 patient. Currently, 120 (83%) are alive in CR, 10 (7%) are alive with disease, 3 (2%) died of disease, 1 each died of TRM and other cause, pending response evaluation in 5 (3.4%) and not evaluated due to lost to follow-up in 5 (3.4%). 21 (14.5%) patients had HDC auto-SCT; 18 (86%) are alive in CR, 2 (10%) are alive with disease and 1 (4%) lost to follow-up/unknown disease status. For those 20 (13.8%) patients who received rituximab alone or in combination for relapsed/refractory disease, all are alive; 18 in CR and 2 with the disease. Five-year KM OS is 96% and PFS is 62%. Patients with stage 1-2 vs 3-4 disease had significantly superior median PFS (151 vs 33.6 months, P=<0.001).
Conclusions: In this large cohort from the Middle East, we have seen a relatively higher incidence of NLPHL as compared to other studies from North America and Europe, likely a referral bias. Although treatment failure was significant especially in stage 3-4 patients, salvage treatment with or without HDC auto-SCT resulted in excellent survival. Limited patients received rituximab at the time of treatment failure, their disease control is excellent. This study also demonstrates that patients with NLPHL and adverse features benefited from both rituximab use and HDC auto SCT at treatment failure.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal